Modality Shift Initiatives
Recent breakthroughs in life sciences have accelerated our understanding of biological functions and diseases, paving the way for more efficient and rational drug discovery.
At Kyowa Kirin, we specialize in developing pharmaceuticals that regulate blood components and cellular functions, using recombinant proteins and therapeutic antibodies created with our genetic recombination technologies. We are committed to advancing these biotechnologies and applying them to the rational and effective discovery of new drugs.
Advanced Therapeutic Antibodies
Kyowa Kirin has developed advanced core technologies that enhance antibody properties and production, including our proprietary POTELLIGENT® technology and fully human antibody production technology. We are actively investing in research and development to advance the clinical development of antibody drugs that use our proprietary bispecific antibody technology, REGULGENT®, along with other growth-driving initiatives.
Bispecific Antibody Technology (REGULGENT®)
Unlike conventional Therapeutic Antibodies, which can only bind to one type of antigen, bispecific antibodies are a revolutionary technology that can bind to two types of antigens. This technology enables precise tissue and cell targeting, producing powerful therapeutic effects.
Our R&D Technologies
in Numbers
- Clinical Trials* 5
- Approvals 4
Technologies includes Poteligent, Regulgent,
Full human antibody production technology, ADC.
-
*The number of licensing-out is not included here.
Advanced Antibody Research
At Kyowa Kirin, we build on our extensive research in therapeutic antibody technologies to develop proprietary technologies. By integrating innovative research ideas with in silico drug discovery, we aim to develop advanced antibody technologies with high efficacy and specificity, creating life-changing value.
Hematopoietic Stem
Cell Gene Therapy
Hematopoietic stem cell gene therapy (HSC-GT) has the potential to treat genetic diseases by addressing their underlying causes with a single treatment. This therapy uses hematopoietic stem cells (HSCs), which are distributed throughout the body and continuously supply various blood cells. HSCs can be collected from a patient, modified to correct mutations or add therapeutic functions, and then reintroduced into the body to help control the disease.
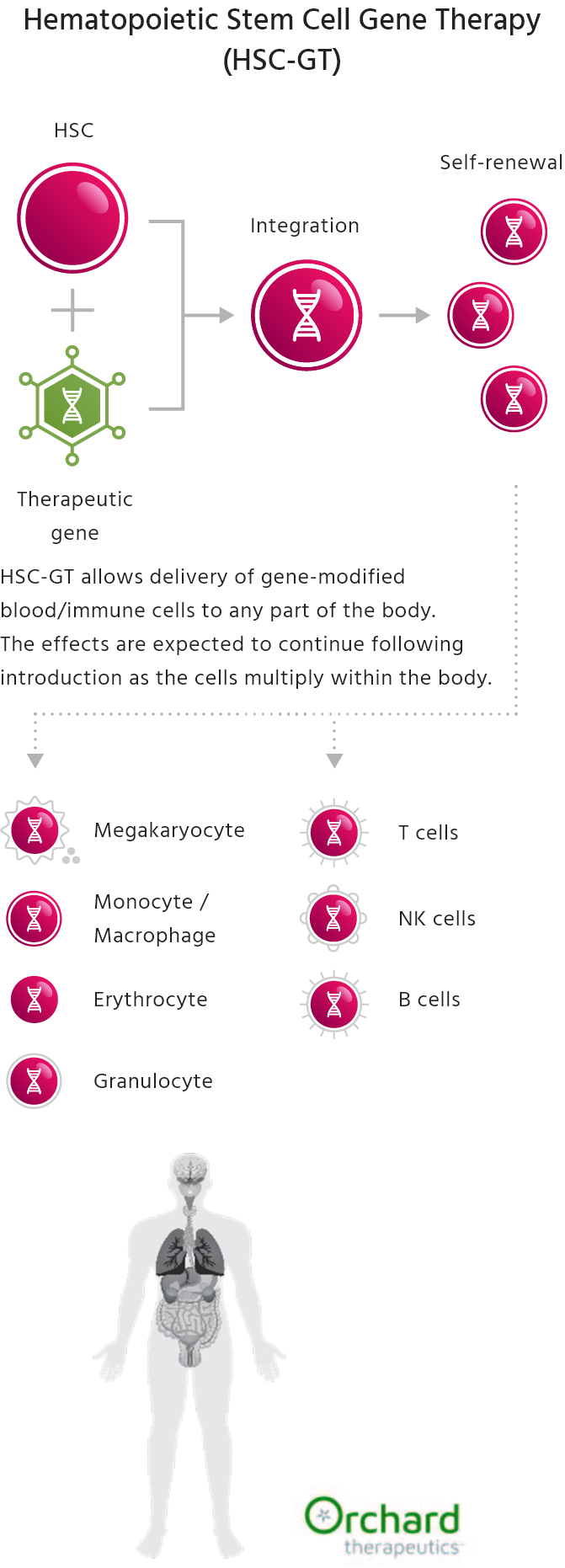
Kyowa Kirin’s Initiatives
in HSC-GT
Pioneering Next-Generation Gene Therapy Through the Fusion of Biotechnology and HSC-GT
Orchard Therapeutics, a Kyowa Kirin company, is a global gene therapy leader focused on ending the devastation caused by genetic and other severe diseases by discovering, developing, and commercializing new treatments that tap into the curative potential of hematopoietic stem cell (HSC) gene therapy. The company has successfully developed and commercialized a gene therapy drug to treat metachromatic leukodystrophy (MLD), a condition that previously had no effective treatment. Currently, we are exploring the potential of HSC-GT to treat mucopolysaccharidosis type I (Hurler syndrome), mucopolysaccharidosis type IIIA (Sanfilippo syndrome type A), and genetic subtypes of frontotemporal dementia and Crohn’s disease.
Additionally, by combining Kyowa Kirin’s strengths in biotechnology with Orchard Therapeutics’ expertise and technology in HSC-GT, we are advancing research into “Beyond HSC-GT,” a next-generation new technology that transcends the boundaries of hematopoietic stem cells and in vitro gene manipulation. In this way, we aim to provide new treatment options for diseases that currently have no effective treatment.

Visit the link below to learn more about Kyowa Kirin’s latest pharmaceutical development pipeline utilizing these cutting-edge technologies.