Ethics and transparency
Compliance
Policy and Strategy
In order to put the 2030 Vision into practice under our philosophy, the Kyowa Kirin Group acts in accordance with its Core Values and with high ethical standards while aiming to become a corporate group trusted by society. Considering compliance as the foundation of all our corporate activities, we have established the framework necessary for compliance, including an organizational structure and basic policies to comply with all laws and regulations, internal and external guidelines and rules and social norms.
Code of Conduct and Group Policies
The Kyowa Kirin Group Code of Conduct sets forth the actions that should be taken by everyone working in the Kyowa Kirin Group. Translated into the local languages of each region, Kyowa Kirin is working to ensure that all employees of the Group around the world are familiar with and understand this Code of Conduct. All executives and employees pledge to comply with the Code of Conduct, and their understanding and compliance is monitored through employee awareness surveys and other means. We also encourage all partners in our supply chain to comply with the Code of Conduct. In addition, basic policies related to individual business areas that must be observed have been established as the Kyowa Kirin Group Policies.
The Code of Conduct and the Group Policies have been established with the approval of the Board of Directors. The Code and Policies are continuously reviewed in light of changes in the external environment, including laws and regulations, as well as changes in the internal environment. Any revisions or repeals are approved by the Board of Directors.
Governance
Compliance Framework
Under the supervision of the Chief Compliance Officer (CCO), the Kyowa Kirin Group has established a Global CSR Head, who is responsible for overall compliance throughout the Group, and a Corporate Social Responsibility (CSR) Management Department to assist the Head in their duties. The Global CSR Head and the CSR Management Department take the leading role in compliance, formulating and implementing compliance measures on a global and regional basis in collaboration with the Regional CSR Heads*1, who are responsible for compliance in the three regions of JAPAC (Japan and Asia Pacific), North America and EMEA. The Kyowa Kirin Group has established regional Compliance committees in the three regions to discuss compliance-related activities. These committees are held on a regular basis to discuss the status of activities and issues that are specific to each region. In addition, a joint Group Compliance Committee meeting encompassing the three regions is held twice a year to deliberate on compliance strategies and actions plans for the entire Group, and to report on the progress of activities during the year. The Group Compliance Committee is chaired by the Company’s Representative Director of the board and Executive Vice President. Important matters discussed in these committees are reported to the Board of Directors.
- *1:Currently, the Global CSR Head also serves as the Regional CSR Head for JAPAC.
Risk Management
Whistleblowing System
The Kyowa Kirin Group has put in place the Compliance Line, whistleblowing system, in order to prevent, detect at an early stage and correct acts that are against the Kyowa Kirin Group Code of Conduct(bribery, improper profit sharing, illegal political contributions, other corrupt acts, harassment, and various human rights violations), as well as acts that seriously damage the brand value of the Kyowa Kirin Group. We have established and operate our whistleblowing system in accordance with the guidelines based on Article 11 of the Whistleblower Protection Act and the Corporate Governance Code, referring to the international standard ISO 37002 issued by the International Organization for Standardization (ISO) based in Geneva, Switzerland. On top of the strict adherence to confidentiality and a rule that those reporting an incident will not be subjected to any retaliation, steps have been taken to establish an internal and external point of contact for reporting that can be accessed by telephone, electronic and postal mail as well as online tools. Reports can also be filed anonymously. In this manner, every effort is being made to create a simple and easy reporting environment. In the case of the internal contact point, the Human Resources Department receives reports. In the case of the external contact point, reports are forwarded from the outsourced external contact point to the CSR Management Department, etc., of the Kyowa Kirin Group in accordance with predetermined rules to initiate an investigation and respond to the matter. We have also introduced a process under which reports concerning directors are passed directly to company auditors. If the reported matter is found to be true by an investigation, corrective measures and recurrence prevention measures will be taken. In some cases, the matter may be referred to the Award and Disciplinary Committee, which will consider whether disciplinary action should be taken. The results of the company’s response to whistleblowing are reported to the Board of Directors and management on a regular basis. Moreover, messages from the CEO on such topics as the importance of the Compliance Line, confidentiality and non-retaliation are sent out on a continuous basis. The point here is to ensure that employees gain a better understanding of the system through group training and e-learning and while maintaining a continuous awareness toward each point of contact for reporting. Details of the Compliance Line are readily available on the Company’s website and posters displayed throughout the workplace. In response to Japan’s revised Whistleblower Protection Act, we were confirming the design and operation of our system, revising internal rules, and making continuous improvements.
Overseas subsidiaries operate local whistleblowing systems in each region. We also established and operate a global line that enables overseas subsidiaries’ employees to report directly to the Group’s head office in Japan in their local language.
In 2024, a total of 36 reports in Japan and overseas were received by the Compliance Line at the Group’s head office.
Internal Audits
At the Kyowa Kirin Group, the internal audit unit conducts risk-based audits from an independent standpoint at each location, including subsidiaries in Japan and overseas. Internal audits verify and evaluate the status of compliance framework establishment and implementation at each site. In addition to pointing out issues and proposing recommendations for improvement, internal audits also help monitor conduct to ensure that appropriate corrective measures are taken. The results of internal audits are presented to the Board of Directors. At the same time, efforts are directed toward strengthening collaboration and improving governance to enhance the effectiveness of audits within the Group by sharing details of the information discussed at regular meetings with company auditors as well as accounting auditors.
Specific Initiatives
Compliance Training
Each region of the Kyowa Kirin Group designates a compliance week or month each year to provide an opportunity to reaffirm the importance of compliance. In Japan, various e-learning and other programs were also held in 2024, including the dissemination of a message from the CCO, and group training for executives and employees.
All executives and employees (including contract, temporary, and part-time employees) of the Group annually attend training to ensure that the Code of Conduct is well understood. Training is conducted globally and held both in person and on an e-learning basis. In 2024, e-learning was conducted with common global content.
Refer to the following for details of other major compliance training conducted during 2024.
Major Compliance Training Results in FY2024*3
You can see this table by scrolling horizontally.
| Category | Details | Scope | Number of Participants | ||
|---|---|---|---|---|---|
| Kyowa Kirin(Non-consolidated) | Group Companies | ||||
| Japan | Overseas | ||||
| Overall training | The Code of Conduct/Anti-Bribery and Anti-Corruption*4*5 | ○ | ○ | ○ | 6,184 |
| Training by employee group | Compliance training for new employees | ○ | 71 | ||
| Training by specific field | Whistleblowing system*4 | ○ | ○ | 4,727 | |
- *3:Training was conducted by the head office in Japan. Region-specific training was conducted via respective region.
- *4:Participants include temporary employees, contract employees, and part-time employees.
- *5:Since FY2024, training has been conducted that integrates the Code of Conduct and Anti-Bribery and Anti-Corruption.
Compliance・Human Rights Awareness Survey
The Company participates in the annual Compliance・Human Rights Awareness Survey implemented by the Kirin Group. The results of the survey help to identify changes in employee awareness and issues that need to be addressed, and are utilized in formulating the Group’s initiatives.
Ensuring Patient Safety and Appropriate Use of Medicines
Pharmacovigilance (PV)
Our Mission
To protect public health by ensuring the safe and effective use of our innovative therapies across the world. We are dedicated to providing timely and accurate information to healthcare professionals, regulatory bodies and our patients.
Our Vision
We strive towards the ideal of patient safety by predicting and preventing adverse reactions through innovative pharmacovigilance practices.

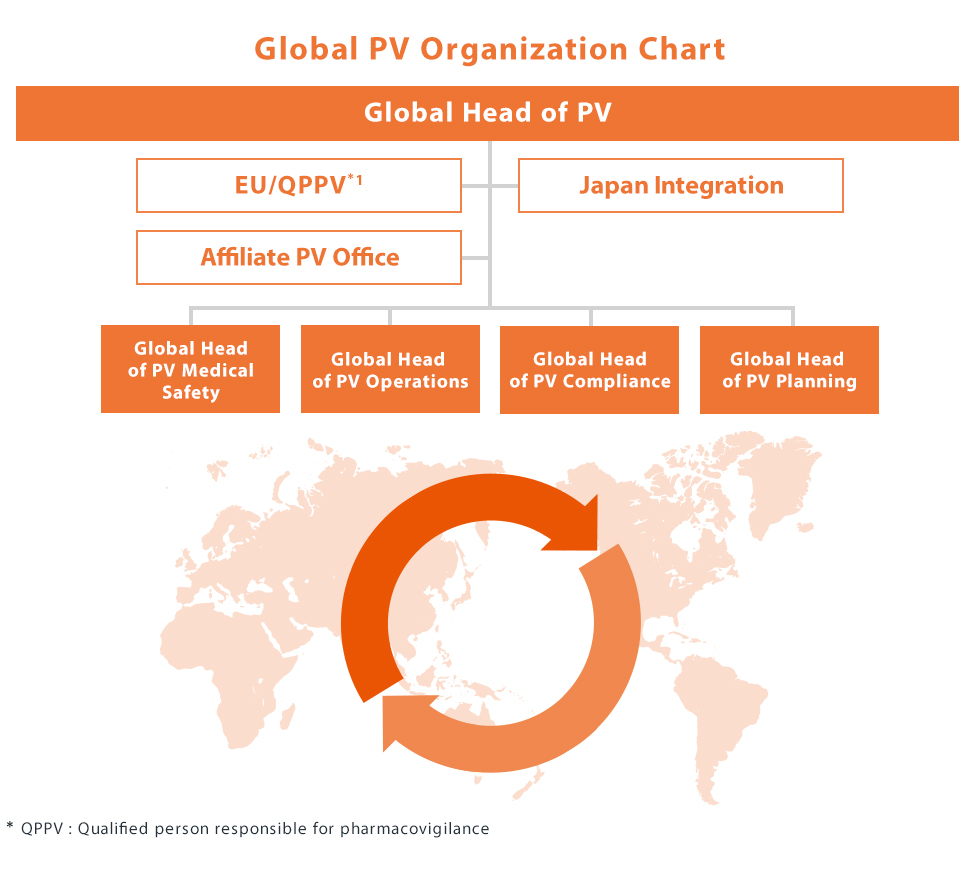
Global Pharmacovigilance Organization
As Kyowa Kirin's innovative therapies reach more patients across the world, the pharmacovigilance organization has evolved to ensure patient safety continues to be a priority across the product life cycle, from discovery through maturity in all our markets. As a result we have adopted a fully global operating model based on functional pillars (Medical Safety, Operations, Compliance, Planning), which underscores our ethos in working harmoniously across boundaries to serve our patients.
Our Challenges
To achieve our vision, we must:
- Become an agile organization that rapidly responds to changes in the internal and external environment, as we reach more patients across the world and manage a dynamic global regulatory landscape.
- Equip our colleagues with global leadership capabilities to carry the organization into the future.
- Implement innovative platforms to deliver better quality, more efficient and real-time safety analysis for proactive communication to our stakeholders.
Message from Global Pharmacovigilance Head
“Always striving for the ideal of patient safety.”
We safeguard the health of our patients through vigilant monitoring of real-world safety data, identifying emerging trends and potential risks. By collaborating with regulatory bodies, healthcare professionals and patients we create a network of transparent benefit-risk communication. Our global team members work across boundaries, bringing their diversity in challenging the status quo and finding innovative ways of working as we lead into the future. As a patient centric safety organization, we live into our core value of Commitment to Life – doing everything we can to improve the quality of life for people around the world.

Global Pharmacovigilance Head
Promoting Proper Use of Medication
Policy and Strategy
Kyowa Kirin places a priority on providing information to promote the proper use of pharmaceutical products. We believe that it is imperative to promptly share accurate information with patients and medical professionals, who are flooded with miscellaneous information on drugs.
Governance
In addition to sales activities by medical representatives (MRs), we use multiple channels of communication, including a contact center for fielding inquiries on drugs, medical science liaisons (MSLs) and a website catering to medical professionals, in order to provide timely information in tune with the latest changes in the medical environment. The information collected is passed on to relevant divisions, and measures are taken to “foster” drugs by maximizing their product value while assuring their safety and quality.
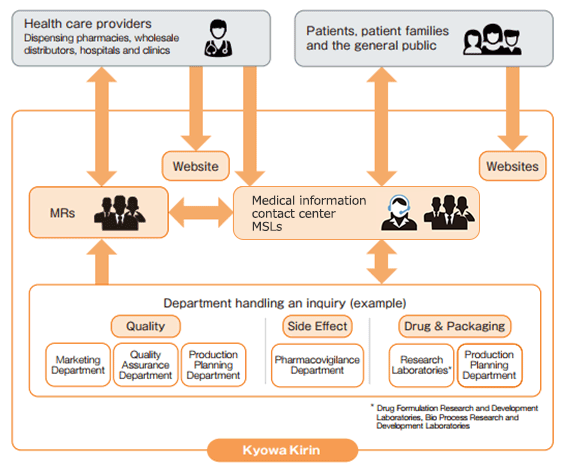
Medical Representatives (MRs)
Based on professional expertise, our MRs provide medical professionals with information highly useful for treating patients. They especially focus on providing information on the efficacies and safety of new drugs, for which medical professionals have both high hopes and doubts, as well as on the new value added to existing drugs due to their expanded indications and other factors.
Medical Information Contact Center
In accordance with the policy advocated by the Japan Pharmaceutical Manufacturers Association, we provide appropriate use information regarding our pharmaceutical products. We are committed to addressing inquiries not only from healthcare professionals but also from patients and the general public who reach out to us by phone. However, please understand that we are unable to respond to inquiries pertaining to individual diseases, treatment methods, or health consultations that require a physician's diagnosis.
Additionally, 'Kyowa Kirin Medical Site©' for healthcare professionals has a 'Product Q&A Chatbot' and 'Frequently Asked Pharmaceutical Product Q&A' available. We aim to provide comprehensive information to assist with the proper use of pharmaceutical products."
Medical Science Liaisons (MSLs)
Medical Science Liaisons (MSLs) leverage their advanced expertise in their assigned therapeutic areas to engage in medical and scientific information exchange with healthcare professionals and provide information that addresses the needs of patients. They operate independently from the sales department (e.g., Medical Representatives) and work daily to promote the appropriate use of pharmaceutical products.
Specific Initiatives
Website: Kyowa kirin medical site®
Through our website "Kyowa kirin medical site®" exclusively tailored to medical professionals, we share detailed product information and disease-related academic meeting reports and publications on nephrology, cardiovascular disease, diabetes mellitus, oncology, blood, immunology/allergy, the central nervous system, and the gastrointestinal regions. Video contents are also offered to facilitate understanding.
Health/disease information websites for patients and the broader public
Ethical Approaches Toward R&D and Production
Policy and Strategy
In each research, development, and production process, the Kyowa Kirin Group is making the following efforts to remain in compliance with domestic and international laws and regulations, internal and external rules and regulations as well as social norms, while fulfilling its legal responsibilities and the ethical responsibilities required by society.
Efforts relating to clinical research
Kyowa Kirin Group Clinical Research Policy
Revised on April 1, 2020
Clinical research on humans is essential to verify the efficacy and safety. In order to contribute to the health and well-being of people around the world by creating new value through the pursuit of advances in life sciences and technologies, at Kyowa Kirin Group, we will conduct research based on the following principles:
- 1.Research significance
We will conduct or support clinical research that contributes to medical care on scientific basis. - 2.Participants protection
We will respect the free will and privacy of the participants and their families in clinical research and protect the dignity and human rights of life. For this purpose, we will provide adequate explanation prior to participation in research activities and we will minimize potential risks and give due consideration to vulnerable populations, including children and the elderly. - 3.Compliance
We will comply with international norms such as the philosophy of the Declaration of Helsinki, applicable laws and regulations, guidelines, and industry codes in each country. Where applicable and appropriate, we will engage in third-party reviews and consider their advice. - 4.Information management
We will strictly manage information of research contents and funds, and will disclose necessary information appropriately and accurately. - 5.Implementation system
We will have appropriate knowledge on science and research ethics and we will conduct research with responsibility and commitment to our mission. We will construct and operate an appropriate organizational system, and compatible business processes. We will also collaborate with appropriate external research institutions to conduct appropriate ethical and scientific research.
In the drug discovery research and development process, the significance of research utilizing human-derived samples is rising, requiring increased focus on ethical considerations. In addition to complying with relevant laws and regulations including the Pharmaceutical and Medical Device Act, the Kyowa Kirin Group conducts education and training for its employees and creates opportunities to acquire third-party certifications and undergo third-party reviews in order to protect human rights and personal information and ensure the reliability of its research findings in line with the ethical principles of the Declaration of Helsinki.
Specific Initiatives
For employees who are engaged in research, Kyowa Kirin Group conducts education and training on related laws and regulations, bioethics, etc.
When conducting research involving human subjects, we provide opportunities for review by a third-party organization such as a Research Ethics Review Committee or Institution Review Board.
Efforts relating to Animal Welfare
The Kyowa Kirin Group has established its Animal Testing Regulation in view of laws, regulations and guidelines pertaining to animal testing. Based on this set of rules, we implement a range of initiatives to ensure that the breeding management of laboratory animals and animal testing are properly conducted in consideration of animal welfare and scientific rationality.
The Rule for Animal Experiments of Kyowa Kirin Co., Ltd. (in Japanese only)
Established on October 1, 2008
Revised on May 1, 2025
Specific Initiatives
Kyowa Kirin conducts proper animal testing and in-house inspection based on the Ministry of Health, Labour and Welfare’s guidelines and international guidelines. Furthermore, in November 2013, the Association for AAALAC International, a global third-party accreditation body, visited the Tokyo Research Park and Fuji Research Park to conduct surveys, and the two research sites obtained full certification in March 2014.
Efforts relating to Biotechnology (Including Biohazards and Environmental Protection)
In creating pharmaceuticals, the Kyowa Kirin Group advances its own research by means of advanced technologies and unique perspectives while developing and providing high-quality products. As a representative life science company of Japan with firm foundations in biotechnology, we will continue to contribute to the health and lives of people around the world.
To use biotechnology techniques that show consideration for environmental and safety aspects, we are complying with the Act on the Conservation and Sustainable Use of Biological Diversity through Regulations on the Use of Living Modified Organisms (Cartagena Act) and, having put in place an internal committee, are managing the situation in an appropriate manner.
So that genetically recombined material is not released into the outside world, we are advancing measures designed to prevent environmental pollution by proper handling and being sure that such material has been inactivated prior to disposal.
To verify whether these initiatives are being implemented in an appropriate manner, we are performing regular monitoring activities at each business site. We are committed to ongoing risk minimization by sharing the results of these activities and any near-miss incidents that have occurred at business sites with the committee and applying them to countermeasures across business sites.
Efforts to Prevent Research Misconduct
Kyowa Kirin Group has established regulations for dealing with misconduct in research activities, and is working to prevent misconduct in research activities.
Efforts relating to Intellectual Property
Policy
Research and development (R&D) are the business foundation of the Kyowa Kirin Group, and therefore, intellectual property (IP) is an important business asset for us. For IP that is an essential part of our R&D and business strategies, we strive to maximize our product value through lifecycle management as well as acquire and maintain effective patent rights through strategic applications. At the same time, we endeavor to ensure freedom in our research and business activities while respecting the rights of other parties and promoting compliance so as not to infringe them, and we will respond promptly and appropriately to any IP dispute that may arise. We believe these activities contribute to maximizing the value of our global business activities, maximizing profits and minimizing risks, resulting in a stable supply of pharmaceuticals.
Specific Initiatives
Respect for Human Rights
Policy and Strategy
Message from Executive Officer in Charge of Human Resources & General Affairs Department, Kyowa Kirin Co., Ltd.
Shoko Itagaki
Chief People Officer (CPO) & Global Human Resources Head
The "Guiding Principles on Business and Human Rights" formulated by the United Nations emphasize "respect for human rights" as a value that companies should uphold. In the Kyowa Kirin Group, as we expand our business globally to achieve our 2030 vision, the number of our stakeholders has significantly increased, and the scope of 'business and human rights' has broadened. We have established a cross-organizational working team and, with advice from external experts, have formulated the "Kyowa Kirin Group Human Rights Policy" while deepening our understanding of the extensive human rights issues faced by global companies.
This policy applies not only to all executives and employees of our group but also to all individuals working in the value chain related to our group's business, products, and services, and we require compliance with this policy. Upholding one of our core values, "Integrity," and maintaining consistent honesty and ethics, we will continuously advance our human rights due diligence efforts based on this policy. We aim to create smiles for everyone working within our entire value chain.
Human Rights Policy
The UN Guiding Principles on Business and Human Rights require companies to formulate a human rights response policy as their responsibility and to disseminate that policy not only to their employees but also to their various other stakeholders. Previously, the Kyowa Kirin Group had been promoting human rights initiatives in accordance with the Kirin Group Human Rights Policy. In December 2022, however, the Kyowa Kirin Group formulated its own Kyowa Kirin Group Human Rights Policy in light of changes in social conditions and demands from society. Positioned as the superordinate policy for all documents and norms concerning efforts to respect human rights in the Group’s business activities, this policy is applicable to all the Group’s officers and employees. Expecting all our business partners to give us their support in this matter, we will also strive to ensure that our suppliers remain in compliance with this policy.
Human Rights Policy Formulation Process
In receipt of cooperation in its formulation from external experts, the specified non-profit organization Caux Round Table Japan (CRT Japan), we subjected the Policy to repeated text brush-ups and multiple reviews, for example in human rights due diligence workshops, as well as by our parent Kirin Holdings Company, Limited, chief HR officers from overseas subsidiaries, and the Compliance Committee*.
- * :The committee’s former name was the CSR Committee.
Governance
In July 2017, we established the Kyowa Kirin Group Human Rights Enlightenment Promotion Committee. This committee formulates, promotes, and supports plans for human rights enlightenment. The committee also provides support for investigations and cooperation for the prompt resolution of human rights issues, if and when they arise. The Committee is chaired by the Director of the Human Resources Department of the Company and consists of Directors of the relevant divisions and heads of the organizations in charge of human resources at major subsidiaries. Kyowa Kirin's Human Resources Department serves as the secretariat.
In daily operations, Kyowa Kirin's Human Resources Department oversees general matters concerning human rights, but as human rights issues are wide-ranging, the department conducts its initiatives in cooperation with other departments and Group companies. The status of compliance with all laws and regulations, including those related to human rights, is reported at the Compliance Committee (chaired by the Chief Compliance Officer), which meets four times a year, to check the effectiveness of our measures, and the result is reported to the Board of Directors.
Risk Management
Human Rights Due Diligence
Human Rights Due Diligence Working Team Activities
In accordance with the full-scale globalization of our business, the impact of our activities on rights-holders (those facing human rights issues) around the world is expanding. The co-creation of value with stakeholders is essential in the provision of life-changing value. In doing so, we believe that it is necessary to consider the human rights of not only our employees but also of those related to our business partners. Having launched a working team, consisting of Corporate Planning Department, CSR Management Department, Procurement Department and Human Resources Department in 2022 to fulfill the corporate social responsibility that is respect for human rights, we are commencing initiatives with regard to human rights due diligence. Having obtained CRT Japan’s assistance, under the United Nations Guiding Principles on Business and Human Rights and based on the demands of society and the laws and regulations of each country, we are promoting the establishment of an internal system to fulfill our responsibility to respect human rights as a company. Having received multiple proposals from CRT Japan, we are progressing with them one by one. In 2022, we (1) formulated the Kyowa Kirin Group Human Rights Policy and (2) held study sessions on business and human rights and workshops to identify human rights issues (human rights due diligence workshops) for the relevant departments.
Holding of Human Rights Due Diligence Workshops/Kyowa Kirin Initiatives Designed to Identify Human Rights Issues
In August 2022, we held an online Human Rights Due Diligence Workshop for the purposes of: (1) Understanding the current situation and acquiring knowledge about business and human rights; (2) Identifying the human rights issues faced by Kyowa Kirin; and (3) Reviewing the above-mentioned Kyowa Kirin Group Human Rights Policy. (Participating departments: Strategy Division, Production Division, R&D Division, Pharmacovigilance Division, SCM Department, Corporate Communications Department, CSR Management Department, Procurement Department, Human Resources Department).
At the workshop, opinions were exchanged on, for example, current initiatives, potential human rights themes, rights-holders, developments in the value chain (to what stages in the value chain do human rights issues apply).
With regard to the human rights issues extracted at the workshop, we confirmed the status of awareness and efforts to address issues in all Kyowa Kirin departments while the working team prioritized the human rights issues that need to be addressed and promoted initiatives. We also identified high-priority issues not only by our own evaluation but also by factoring in the level of interest from society (evaluations by specialized institutions). In 2023, we conducted a survey on foreign technical interns for suppliers at the Takasaki Plant. We also directly interviewed the administrative departments and technical trainees of our suppliers (Shin Nippon Wex Co., Ltd., (Link![]() )) who are actually accepting skilled trainees. As a result, the company confirms that human rights of skilled trainees are respected and that there were no particular concerns about human rights violations. The report is then fed back to the company.
)) who are actually accepting skilled trainees. As a result, the company confirms that human rights of skilled trainees are respected and that there were no particular concerns about human rights violations. The report is then fed back to the company.
We will continue to investigate and evaluate human rights issues in the supply chain.
Human Rights Due Diligence
Kyowa Kirin is endeavoring to ensure human rights throughout its pharmaceutical value chain.
Human rights considerations in research
Kyowa Kirin sets internal rules with the aim of assuring ethical and scientific validity in human genome analysis and research using human tissue as well as preventing the loss of dignity and protecting the human rights of tissue donors.
Safeguarding human rights in clinical trials
When conducting a clinical trial involving human subjects, Kyowa Kirin, pursuant to the Declaration of Helsinki, gives top priority to protecting the human rights and personal information of patients participating in the trial, securing their safety, and giving consideration to their welfare, and complies with the laws and regulations and Good Clinical Practices (GCP) guidelines of the applicable countries.
Ensuring human rights in purchasing activities
We have formulated the "Kyowa Kirin Group Procurement Policy" and the "Kyowa Kirin Group Supplier Code of Conduct" to help us ensure human rights throughout the supply chain in collaboration with our suppliers.
Ensuring human rights in engaging with medical institutions and patient organizations
Pursuant to Kyowa Kirin Group Policy for the Promotion of Pharmaceuticals and Interactions with Healthcare Professionals, Healthcare Organizations and Patient Organizations, we act with high ethical standards and in the best interest of patients when we engage with medical institutions and patient organizations.
Internal Whistleblowing System
We have an internal reporting contact in place, to which employees can turn when they face human rights issues. When a report is filed, Human Resources Department and the CSR Management Department investigate the facts and take appropriate measures while protecting the informant and the confidentiality of the report. Furthermore, we have also established various contacts for other issues (including harassment, mental health, disabilities, and LGBTQ issues) to support those who face them.
Internal Whistleblowing System
Complaints and reports related to human rights (Whistleblower contact for a wide range of stakeholders)
The Group accepts human rights-related complaints and reports from all stakeholders in its supply chain through the engagement and remedy platform provided by the Japan Center for Engagement and Remedy on Business and Human Rights (JaCER), which is based on the United Nations Guiding Principles on Business and Human Rights.
- We are committed to resolving essential human rights issues by accepting complaints through a third party, thereby ensuring equality and transparency in the handling of complaints, and responding appropriately to complaints and whistleblowing.
- With respect to whistleblower's reports we receive, we will investigate the facts of the report, engage in necessary dialogue regarding the report, and implement corrective measures (including prevention of recurrence and response to objections).
- In responding to the whistleblower's report, the Company shall ensure that the whistleblower will not be disadvantaged and shall protect his/her personal information. Unless there is a justifiable reason to do so, such as when required by law, we will not use the information related to the case for any other purpose.
For whistleblowing through JaCER, information will be disclosed anonymously on JaCER's website on a regular basis.
Internal Reporting System
Specific Initiatives
Training Programs and Awareness Activities
In order to establish the concept of respecting human rights based on our human rights policy, we conduct various training sessions for our executives and employees. In 2022 and 2023, we rolled out human rights awareness training and training related to business and human rights for our domestic employees. In 2024, we implemented e-learning using global common content.
We have taken various measures against the issue of harassment as one of the human rights issues, and are working to improve the environment and corporate culture to create a harassment-free environment.
Specifically, we send out a message by our CXO during the "Month for Eliminating Harassment," and conduct awareness-raising training for all employees. We continuously incorporate content related to harassment prevention in training based on rank such as new employee training and newly appointed managerial staff training, providing knowledge on how to prevent harassment according to job rank and position.
In addition, we are a member of the Business Ethics Research Center, and participate in the Harassment Study Group and the Diversity Management Study Group to acquire relevant knowledge and improve understanding. As a means of reviewing our measures, we conduct on an annual basis the Kirin Group Compliance/Human Rights Awareness Survey in conjunction with the entire Kirin Group. By analyzing the survey results, we identify changes in our employees' awareness and issues that need to be solved, which helps us implement effective initiatives within our Group.
Response to the UK Modern Slavery Act
Kyowa Kirin International plc, an overseas group company of Kyowa Kirin, discloses the status of its human rights initiatives related to modern slavery risks in its business activities and supply chain in accordance with the UK Modern Slavery Act.
Recruitment and Benefits
Kyowa Kirin International plc, an overseas group company of Kyowa Kirin, discloses the status of its human rights initiatives related to modern slavery risks in its business activities and supply chain in accordance with the UK Modern Slavery Act. Seeking to hire people who take pride in working to save lives as well as those with global competence, Kyowa Kirin pursues fair recruitment without discrimination on the basis of gender, nationality, disability or other traits.
In addition, Kyowa Kirin supports the daily lives of its employees and their families through a variety of benefit programs. We also apply the programs to non-regular employees such as contract workers, including medical care such as health checkups, childcare leave, recreational support, use of benefit enhancement plans, and other benefits.
Employee Health and Safety (Occupational Health and Safety)
Employee Health and Safety (Occupational Health and Safety)
Policy and Strategy
Kyowa Kirin Group Policy for Occupational Health and Safety
Established on November 22, 2018
We at the Kyowa Kirin Group (hereafter referred to as "Our Group") believe that ensuring the health and safety of all its employees is crucial in fulfilling its management philosophy of "striving to contribute to the health and well-being of people around the world by creating new value through the pursuit of advances in life sciences and technologies." Therefore we engage in ongoing activities to prevent occupational accidents and disasters. Our aim is to foster a deeply rooted culture that encourages each and every employee to think and act on their own accord so that the standards of Our Group's occupational health and safety can be enhanced.
- 1.By operating our management system for occupational health and safety, we promote ongoing improvements with the aim of enhancing Our Group's health and safety standards.
- 2.We strive to prevent occupational accidents and disasters by identifying and assessing sources of hazards in workplaces and taking measures to eliminate or reduce the identified risks.
- 3.We comply with laws and regulations concerning occupational health and safety in the countries and regions where Our Group conducts business activities, and make ongoing efforts to ensure the health and safety of our employees.
- 4.We implement education, training and awareness activities on occupational health and safety in a rigorous and systematic manner with the aim of raising employee awareness.
- 5.We actively encourage communication between the employer and each employee indispensable for ensuring employee health and safety, and implement health and safety activities engaging all employees.
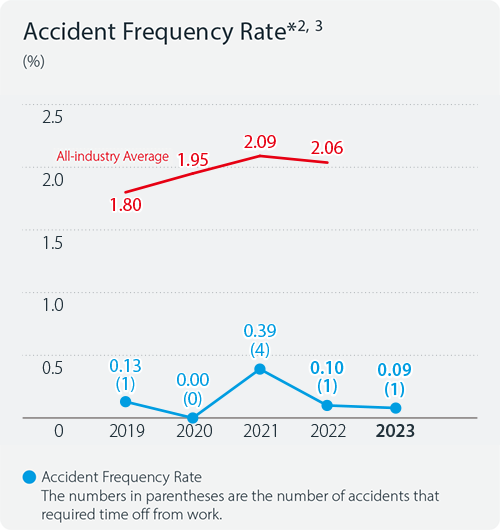
Indicators and Targets
Accident frequency rate*1
In 2024, the number of lost-time injuries at the Kyowa Kirin Group was zero. The frequency rate*2 and severity rate*3 of occupational injuries were also both zero.
- *1The data for 2020 covers all business sites of Kyowa Kirin Co., Ltd. From 2021 and onward, data covers all of the Group’s domestic business sites and overseas production and research sites.
- *2Accident frequency rate: The number of fatal and lost time accidents per million working hours
- *3Accident severity rate: The number of lost days per 1,000 working hours
Specific Initiatives
Implementation of safety culture assessment
In September 2024, we implemented a safety culture assessment across the entire Kyowa Kirin Group in Japan. The results of this assessment, which was performed by an external consultant, indicated that the Group’s overall safety culture level had improved through voluntary initiatives at production and research sites compared to 2021, when the previous assessment was conducted. Going forward, the Group will continue to implement safety initiatives at each site, further enhancing the development of its safety culture.
Safety management training for newly appointed managers
Since 2007, Kyowa Kirin has been conducting safety management training for newly appointed managers, as stipulated by the Industrial Safety and Health Act. This training targets newly appointed managers of plants and research centers, annually providing programs that cover the Group’s occupational health and safety policy and safety education as well as specific methods of safety activities, among others. To date, a total of 668 managers have received the certification and are now taking the lead in promoting safety activities to help create an environment where employees can work in a secure place.

Safety training using VR
The Ube Plant conducted a risk experience training program using VR technology, aiming to raise individual employees’ sensitivity to danger. In addition to watching videos about tripping, slipping, and falling caused by smartphone use while walking, the technology allowed the users to experience these accidents in a more realistic way through physical sensations such as tilting, shaking, and impact synchronized with the videos.
Around 80 employees, including the plant manager, have experienced the physical feedback provided through the training, which has helped improve their safety awareness in their daily work. We proactively incorporate the latest technology and information into our safety programs, and continue to promote a strong safety culture.

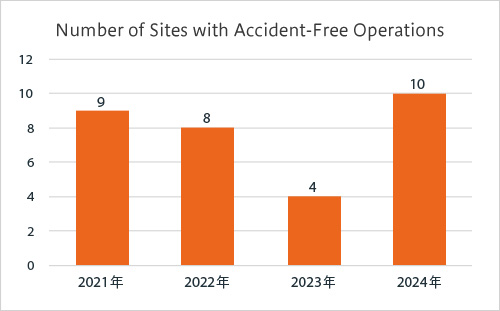
Commendation for sites with accident-free operations
The Kyowa Kirin Group commends sites that have had no occupational or commuting-related accidents throughout the year with the Accident-Free Operations Award. In 2024, 10 out of 18 sites received the award. Each of the Group’s sites works to prevent accidents through consistent health and safety activities, contributing to stable daily operations.
Initiatives for safe driving
Kyowa Kirin has an internal safety system to promote traffic safety, under which traffic accidents and violations by employees are analyzed and compiled as reference materials by the head office administrative department. Each sales office has designated Traffic Safety Promotion Committee members who promote traffic safety awareness while supporting Safe Drive Managers. The results of these activities are reported to the head office administrative department, which helps facilitate safe driving initiatives through mutual communication between the department and the offices.
Meanwhile, all leased company vehicles have been replaced with models equipped with an autonomous emergency braking system (AEBS). In addition, the fleet is transitioning to models featuring around-view monitors to help prevent accidents in parking lots and other locations. Furthermore, we require all drivers to undergo alcohol detection checks before and after work, thereby ensuring compliance and protecting employees.
We also commend sales offices that have had no accidents or violations throughout the year as Outstanding Offices in Traffic Safety. In 2024, 26 sales offices have been awarded the commendation nationwide.

Business Partner Initiatives
Policy and Strategy
Kyowa Kirin Group’s initiatives regarding business partners are based on the Kyowa Kirin Group Business Partner Management Policy. For more information about collaboration with suppliers , please visit “Sustainable Procurement” page.
Kyowa Kirin Group Business Partner Management Policy
Established on August 1, 2025
I. PURPOSE
The philosophy of Kyowa Kirin Group is “to strive to contribute to the health and wellbeing of people around the world by creating new value through the pursuit of advances in life sciences and technologies”.
Under this philosophy, we aim to achieve the vision of “the successful creation and delivery of life-changing value that ultimately makes people smile”.
To realize this vision, we encourage not only our employees but also our Business Partners to act in accordance with the spirit of the “Kyowa Kirin Group Code of Conduct”.
This Group Policy sets forth specific guiding principles to encourage our Business Partners, who are one of our important stakeholders, to adhere to the standards outlined in the “Code of Conduct”.
II. SCOPE
This Group Policy shall apply to everyone working in Kyowa Kirin Group and its subsidiaries (hereinafter, “Kyowa Kirin Group”), regardless of country or regions, or position or title, such as officer, employee, fixed-term employee, or temporary staff, or full-time or part-time.
III. DEFINITIONS
“Business Partner”: Individuals or organizations that conduct business with Kyowa Kirin Group, specifically those:
- ithat provide goods or services directly or indirectly to Kyowa Kirin Group
- iithat purchase goods or services directly or indirectly from Kyowa Kirin Group. This includes, but is not limited to, Suppliers, Distributors, Research Partners, Development Partners, and Sales Partners, as well as Agencies acting on Kyowa Kirin Group’s behalf. It also includes subcontractors and agents of these partners. However, this does not include individuals such as Patients who are the end consumers of the products or services.
“Due Diligence”:The process that Kyowa Kirin Group should implement to identify, prevent, and mitigate actual and potential negative impacts on its business and other business relationships, and to fulfill its responsibility to explain how it will address these negative impacts. Specifically, this involves identifying and assessing risks, implementing measures to prevent and mitigate risks according to their severity, and disclosing the measures implemented to stakeholders in an appropriate manner.
IV. GUIDING PRINCIPLES
- 1.The Kyowa Kirin Group will encourage and request its Business Partners to act* in accordance with the spirit of the “Kyowa Kirin Group Code of Conduct” through Due Diligence.
- *Actions related to Human Rights, Environment, and Compliance (including Information Security, Prevention of Bribery and Corruption), etc.
- 2.The Kyowa Kirin Group shall strive to strengthen the internal structure to conduct ongoing Due Diligence through clarifying the organizations responsible for each process.
Specific Initiatives
Joining the PSCI
In June 2024, the Kyowa Kirin Group joined the membership of the Pharmaceutical Supply Chain Initiative ("PSCI"), a non-profit organization working as one voice for the pharmaceutical industry to deliver responsible value chains.
Through the PSCI, we collaborate with industry leaders and participate in good practice of sustainable procurement in the pharmaceutical industry. In addition, in our effort to build a more efficient and effective auditing system, we will utilize the results of supplier audits conducted by PSCI member companies, which are shared through the PSCI Shared Audit Program.

Anti-Bribery and Anti-Corruption, Responsible Promotion, Tax Compliance
Anti-Bribery and Anti-Corruption
Policy and Strategy
Acts of corruption hinder the sustainable development of society. The United Nations and the Organisation for Economic Co-operation and Development (OECD) as well as national governments around the world have urged global companies to strengthen their measures to prevent such conduct. Acts of corruption are not tolerated under any circumstances at the Kyowa Kirin Group.
To clarify its strong determination to prevent bribery and corruption, the Kyowa Kirin Group explicitly states in the Kyowa Kirin Group Code of Conduct, which serves as the guiding principles for all who work for the Group, that it prohibits bribery, unjust provision of benefits, illegal political donations, and other acts of corruption. In addition, the Kyowa Kirin Group Anti-Bribery and Anti-Corruption Policy has been put in place to complement the Code of Conduct, along with internal regulations that set forth concrete operational procedures. Meanwhile, Facilitation Payments are prohibited under the Group Policy, even if permitted under the applicable laws, regulations, guidelines or relevant industry codes in the country where the Group company operates.
The Code of Conduct has been translated into languages of the regions in which the Group conducts business and is disseminated across the entire Group together with the Group Policy. The Code of Conduct and the Group Policy are posted on internal and external websites so that they can be viewed by all employees in Japan and overseas.
Kyowa Kirin Group Anti-Bribery and Anti-Corruption Policy
Established on December 13, 2013
Revised on January 1, 2026
- Ⅰ.PURPOSE
The Kyowa Kirin Group (the "Group") aims to be a corporate group that society can trust, while operating in line with its Management Philosophy, Core Values and Vision and through its strict adherence to high ethical standards. As part of this effort, with full recognition of the fact that bribery and corruption are major obstacles to sustainable development of society, we maintain business relationships with stakeholders in compliance with laws and regulations and also in accordance with high ethical standards, and at the same time, engage in fair, transparent and free competition and appropriate transactions. The Group’s position on anti-bribery and anti-corruption is described in the Kyowa Kirin Group Code of Conduct, and we hereby specify our anti-bribery and anti-corruption guiding principles. - Ⅱ.SCOPE
This Group Policy applies to everyone working in the Group, regardless of country or region, position or title, such as officer, employee, fixed-term employee or temporary staff, or full time or part time (the “Employees of the Group”). We will also require all of our business partners or agents to act in accordance with the underlying principles set forth in this Group Policy. - Ⅲ.DEFINITIONS
- 1.“Bribery and Corruption” means (i) offering, promising, providing, authorizing, accepting or requesting anything of value, including improper payments, goods, entertainment, or other improper financial advantages that exceed what is considered appropriate under the applicable laws, regulations, guidelines or relevant industry codes in the country or region where the Group company operates and (ii) making Facilitation Payments (as defined hereinafter).
- 2..“Facilitation Payments” means small payments made to a government official, etc. to expedite or secure a routine governmental action.
- Ⅳ.GUIDING PRINCIPLES
We will abide by the following anti-Bribery and anti-Corruption guiding principles:- 1.We will fully understand the underlying principles of and comply with applicable anti-Bribery and anti-Corruption laws, regulations, guidelines or relevant industry codes in the countries where we operate and do business.
- 2.We maintain a zero tolerance policy against all forms of Bribery and Corruption.
- 3.We strictly prohibit any form of Bribery and Corruption against any party. All Facilitation Payments, even if permitted under the applicable laws, regulations, guidelines or relevant industry codes in the country where the Group company operates, are prohibited under this group policy.
- 4.We require our business partners or agents not to engage in any Bribery and Corruption.
- 5.We will refuse to continue doing business with any business partner or agent if we become aware of any Bribery and Corruption committed by such business partner or agent in connection with the Group’s business.
- 6.The Employees of the Group are required to report immediately any known violations of this Group Policy, including those of applicable laws, regulations, guidelines or relevant industry codes.
- 7.The President and CEO of Kyowa Kirin Co., Ltd. is responsible for the Group's anti-Bribery and anti-Corruption program, and will appoint anti-Bribery and anti-Corruption officers at each Group company for the execution of necessary anti-Bribery and anti-Corruption measures.
- 8.We will keep accurate records of all payments made to and business transactions with the parties concerned.
Governance
Kyowa Kirin’s Chief Compliance Officer (CCO) supervises the Group’s initiatives to prevent bribery and corruption. Under the CCO’s leadership, the Group Compliance Committee and its secretariat, the CSR Management Department, play a central role in executing the initiatives with managers assigned to the task at each Group company. In addition, the audit unit, which is independent from the business execution lines, audits the activities undertaken to mitigate the risks associated with bribery and corruption. The results of these audits are reported to the Audit & Supervisory Board members and the Board of Directors. The Board of Directors oversees the status of implementation of the Code of Conduct and Global Policy including the prevention of bribery and corruption, and their revision is subject to approval of the Board of Directors.
Risk Management
The Group implements a series of ongoing risk management activities, which comprise identifying, analyzing, and evaluating risks, taking measures against risks, checking how risks are addressed, and improving the measures. We recognize that bribery and corruption poses risks that could impact our business management. As far as transferring value to healthcare professionals and others is concerned, we have established a pre-screening and approval process to ensure that an honorarium is set at fair market value and the provision of such benefits as food and beverages is in accordance with industry rules. In addition, we conduct regular risk assessments, and for subsidiaries and departments with a high risk of bribery and corruption, we put in place specific measures in our business plan and monitor progress of initiatives. The CSR Management Department reports updates on risk management at periodic Group Risk Management Committee meetings, checks its effectiveness, and reports the result to the Board of Directors.
Indicators and Targets
In 2024, no fines, penalties, or settlements were imposed on the Group for the violation of anti-bribery or anti-corruption laws, and no disciplinary dismissal resulted from the violation of such laws.
Specific Initiatives
All executive and employees, including contract, part-time, and temporary employees, annually undertake a mandatory anti-bribery and anti-corruption e-learning program, which is available in 8 languages. In addition to the president's message and the contents of the Global Policy and internal regulations, the program includes social trends such as the latest case studies, legal and regulatory developments, and after completing the program, participants commit to comply with the rules. In FY2024, 6,184 executives and employees participated in the program.
The Group offers multiple consultation hotlines to enable any executive or employee to voice any doubts or concerns regarding bribery or corruption with a sense of security. One of them is the Compliance Line, which serves as a contact point for discussing or reporting compliance matters in general including bribery and corruption. Executives and employees can remain anonymous when contacting the Line, their reporting will be kept confidential, and whistleblowers will not suffer any disadvantages as a result of their action.
The Group sets forth the Supplier Code of Conduct, which stipulates the actions and behavior that suppliers (including intermediaries) are expected to adhere to, including those concerning bribery and corruption. We hold briefing sessions for suppliers*1 as an opportunity to regularly explain this "Supplier Code of Conduct" and other guidelines as a part of efforts to promote communication. We conduct questionnaire surveys of new suppliers, as well as of existing suppliers, before entering into transactions with them to assess their adherence to the Supplier Code of Conduct. In view of the survey results, we perform due diligence*2 and strive to reduce the risks related to bribery and corruption.
- *1:
- *2:Duty of care and effort that companies are required to exercise as a matter of course. In this case, due diligence indicates investigating suppliers’ risk of becoming involved in bribery or corruption.
Responsible Promotion
Policy and Strategy
As a corporate group undertaking business that is deeply connected to people’s lives and health, the Kyowa Kirin Group must strive to engage in promotional activities by consistently upholding a high standard of ethics.
To articulate its commitment to ethical promotional activities, the Group has established the Kyowa Kirin Group Policy for the Promotion of Pharmaceuticals and Interactions with Healthcare Professionals, Healthcare Organizations and Patient Organizations in line with relevant laws and regulations of each country in which it conducts business, codes of conduct and guidance set by the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) and regional industry associations.
Kyowa Kirin Group Policy for the Promotion of Pharmaceuticals and Interactions with Healthcare Professionals, Healthcare Organizations and Patient Organizations
Established on January 1, 2024
Revised on January 1, 2026
- 1.PURPOSE
We at the Kyowa Kirin Group (hereafter referred to as "Our Group") seek to fulfill our management philosophy which reads: "Our Group strives to contribute to the health and well-being of people around the world by creating new value through the pursuit of advances in life sciences and technologies". We believe that collaboration with stakeholders is indispensable to accurately understand and help address medical and health-related issues.
Especially, we aim to advance healthcare-related discoveries, enhance the quality use of medicines, and facilitate patient-participatory medical care. To achieve this, we collaborate, partner and interact with patient organizations, consisting of patients, their families and carers, as well as medical personnel and institutions (collectively referred to as the “Medical Community”). Patient-participatory medical care involves collaboration among patients, families and medical personnel and allows patients themselves to actively participate in their own medical care, for example by being involved in decision-making. This leads to high-quality medical care.
We will comply with this Group Policy and promote pharmaceuticals and interact with the Medical Community and patient organizations in the best interest of patients. “Promotion" means any activity undertaken, organized or sponsored by Our Group, which is directed at HCPs, to promote the prescription, recommendation, supply, administration or consumption of its pharmaceutical product(s) through all channels of communication.
- 2.SCOPE
This Group Policy applies to everyone working in Our Group, regardless of country or region, position or title, such as officer, employee, or temporary staff, whether full or part time. - 3.GUIDING PRINCIPLES
- 3.1.Promotion and interaction principles
In line with our Commitment to Life, we will apply high ethical standards to our actions and will comply with the Kyowa Kirin Group Compliance Policy for the promotion of Pharmaceuticals and in our interactions with the Medical Community and patient organizations.- In order to gain or maintain the trust of society, we will be transparent in the type and nature of the relationships we establish with the Medical Community and patient organizations. We will disclose and mitigate conflict of interest situations.
- We will not provide or offer anything in a manner or under conditions that may improperly affect patient-related decisions and/or the independence of decision-makers (e.g., the Medical Community). We will not provide gifts or make payments, in return for certain benefits, nor will we provide facilitation payments.
- We will interact with healthcare professionals aiming to benefit patients and enhance the practice of medicine, by focusing on providing valid scientific and educational information.
- Our Group’s employees are required to comply with this Group Policy regardless of the existence of any relevant laws, guidelines, or industry rules in any country or region where we operate.
In the case that there is a difference between this Group Policy and the relevant laws, guidelines, or industry rules in each country or region, the stricter rules must be followed.
- 3.2.Promotion of pharmaceuticals
- We engage in lawful, code-compliant and ethical promotion. We will provide timely and truthful information that is scientifically and clinically significant when promoting our products, and will never pursue profit through inappropriate promotion or illegal marketing practices.
- We will not engage in any false or misleading promotion or misrepresentation, nor will we disparage competitors’ products. We will promote the proper use of pharmaceuticals in an accurate, fair and balanced manner by presenting the characteristics of pharmaceuticals objectively and without exaggeration.
- We will clearly separate promotional activities and non-promotional activities.
- 3.3.Interactions with patient organizations
- We will establish collaborative, professional, respectful and effective relationships with patient organizations. These relationships will be based on mutual understanding and trust, and aligned with ethical standards. We will respect the independence of patient organizations, and will interact with them for educational and scientific purposes, or to provide support as appropriate.
- 3.4.Transparency of relationships with the Medical Community and patient organizations
- We will ensure high ethical standards in our relationships with the Medical Community and patient organizations. To be accepted as a reliable corporate group in society, we will disclose information on transfer of values to the Medical Community and patient organizations in countries/regions where we operate in an accurate and timely manner and in compliance with the relevant laws, guidelines and industry rules.
- 3.5.Compliance with laws, etc. and establishment of necessary structures
- When promoting our products and interacting with the Medical Community and patient organizations, we will comply with this Group Policy, all applicable laws, guidelines and industry rules in countries or regions where we operate. We will follow the IFPMA code when conducting activities in countries or regions that do not have proper laws and regulations or industry codes. Further, we will also require parties we interact with to comply with this Group Policy, all applicable laws, guidelines and industry rules in countries or regions.
- We will establish and operate appropriate organizational structures and business processes, including education and monitoring related to the promotion of pharmaceuticals and interactions with healthcare professionals, healthcare organizations and patient organizations, that comply with this Group Policy as well as the related laws, guidelines and industry rules in countries and regions where we operate.
Governance
System for the Appropriate Provision of Information
Kyowa Kirin provides information materials, including those pertaining to its pharmaceuticals and products in related fields, advertisements and materials for patients, only after ascertaining their full compliance with relevant laws and regulations of applicable countries and standards set by industry associations.
In Japan, such information materials are reviewed by multiple relevant divisions independent of the sales division as well as by the Information Materials Review Committee, whose members include third parties. Information materials are reviewed from the viewpoints of their scientific validity and whether they comply with the JPMA Code of Practice, fair competition rules and other external standards. The sales division that uses the applicable information materials is not involved in the review process. All divisions are prohibited from using information materials that have not been reviewed and approved.
In our efforts to comply with the Guidelines for Provision of Sales Information on Prescription Drugs set forth by the Ministry of Health, Labour and Welfare (hereinafter, "Guidelines")*1, we have also established a system for checking that sales information is provided in an appropriate manner.
Specifically, we have a division that supervises our activities for providing sales information by way of monitoring and providing guidance to ensure that they are conducted appropriately. Moreover, when providing information on off-label uses of the Group’s pharmaceuticals or unapproved drugs under development by the Group, we ensure compliance with relevant laws and regulations.
Furthermore, we have in place a committee for supervising our sales information provision activities, whose members include third parties, to oversee and guide such activities. The supervisory department for information material review periodically reports to the committee and seeks advice.
- *1:
Specific Initiatives
Training Programs
Employee training is a key factor in ensuring ethical promotional activities.
Training is offered periodically to employees who create information materials and those who engage in promotional activities. Programs include in-person classes given by sales office managers and members of the CSR Management Department, as well as online-based and e-learning courses.
An annual Compliance Promotion Month has been established, with all employees required to undergo a training program to learn about and better understand the code of practice. In the event of any violation of the rules, additional training is promptly conducted to prevent recurrence.
You can see this table by scrolling horizontally.
| Training organizer | Target | Frequency | Scope of training |
|---|---|---|---|
| Regulatory Affairs Department | Personnel in charge of creating materials | 12 times a year | Knowledge required to create materials in compliance with laws and regulations |
| Sales office manager | Sales & Marketing Division | 12 times a year | Guidelines for Sales Information Provision Activities, fair competition rules |
| CSR Management Department | Sales & Marketing Division | 4 times a year | JPMA Code of Practice, Guidelines for Sales Information Provision Activities, fair competition rules |
| CSR Management Department | All employees of Kyowa Kirin Group (in Japan) | Once a year | JPMA Code of Practice |
Tax Compliance
Policy and Strategy
Kyowa Kirin Group Tax Policy
Established on September 1, 2021
Revised on March 19, 2025
The Kyowa Kirin Group (hereinafter, the "Group") strives to carry out corporate activities in a manner trusted by society, based on our philosophy, vision and core values.
With regards to tax operations, the Group strives to maintain and improve our tax compliance, and tax risk management, through enhancing our global tax governance systems.
The Group has established this tax policy and it is applicable to our entire Group. It will ensure tax transparency and appropriate tax payments.
1. Global Tax Governance
Chief Financial Officer (CFO) is the person ultimately responsible for the Group’s tax governance and tax risk management. The Director of the Finance Department shall supervise the execution of the Group’s tax governance and tax risk management activities. In addition, the Director of the Finance Department shall report on the progress of these activities to CFO and report matters of significance to the Board of Directors via CFO.
The Group shall endeavor to enhance the Group’s corporate value sustainably through establishing this tax policy by resolution at the Board of Directors, by building and maintaining an effective global tax governance structure aligned with our “One Kyowa Kirin” business management structure, and by providing the global oversight of tax initiatives.
2. Tax Planning
The Group shall conduct reasonable tax planning where it is aligned with our business objectives and strategies. Since the Group’s corporate value may be undermined in the event that full consideration from multiple tax viewpoints is not given to any global business plan, the group shall consult with external advisors and ensure that sufficient research and consideration is undertaken in respect of our activities. The Group shall only carry out tax planning activities compliant with the relevant tax laws and regulations in the jurisdictions in which we operate.
The Group shall not conduct excessive tax planning that is deemed to be an act of tax avoidance in light of socially accepted practices, or an act of tax avoidance that is without business substance including use of tax havens.
3. Tax Transparency
The Group shall comply with both the tax laws and regulations of each relevant jurisdiction and the OECD Transfer Pricing Guidelines. The Group shall strive to ensure tax transparency by disclosing appropriate tax information in a timely manner.
4. Tax Compliance
The Group shall keep up-to-date with developments in the tax laws and regulations in each relevant jurisdiction and shall make appropriate tax payments in compliance with them.
5. Transfer Pricing
The Group shall comply with the OECD Transfer Pricing Guidelines and set transfer prices for intercompany transactions in accordance with the arm’s length principle. We shall analyze the functions, assets and risks of each Group company in light of the substance of their business activities, and periodically evaluate whether the profit allocation is appropriate in line with the contribution. The Group shall pay appropriate amounts of tax in each jurisdiction based on the value created by our business activities.
The Group shall prepare transfer pricing documentation in accordance with the relevant transfer pricing regulations in each jurisdiction.
6. Tax Incentives
The Group shall endeavor to improve tax efficiency and enhance our corporate value by appropriately utilizing applicable tax incentives, available in each relevant jurisdiction, based on a proper understanding of the intent behind them.
7. Attitudes toward Tax Risk
In the event that the Group is in an uncertain tax position, such as a transaction having multiple possible tax treatments or differences in opinion with the tax authorities of any jurisdictions concerning a tax treatment that the Group has judged appropriate, the Group shall make investigations into such matters by consulting with external advisors and considering the outputs. Also, in order to ensure certainty, the Group strives to reduce the tax risks associated with uncertainty by proactively seeking rulings by tax authorities where they are available.
8. Relations with tax authorities
The Group shall endeavor to establish cooperative relationships with tax authorities by providing appropriate information in a timely manner and by engaging in constructive discussions, in response to inquiries by the tax authorities.
9. Prevention and elimination of double taxation
The Group shall strive to prevent double taxation levied by multiple jurisdictions on the same economic benefits through conducting tax operations in accordance with this policy. If double taxation occurs, the Group shall strive to eliminate it by taking advantage of tax treaties or mutual agreement procedures available in the relevant jurisdictions.