STORY FOR VISION 2030R&D Strategy
CREATING AND DELIVERING
LIFE-CHANGING VALUE
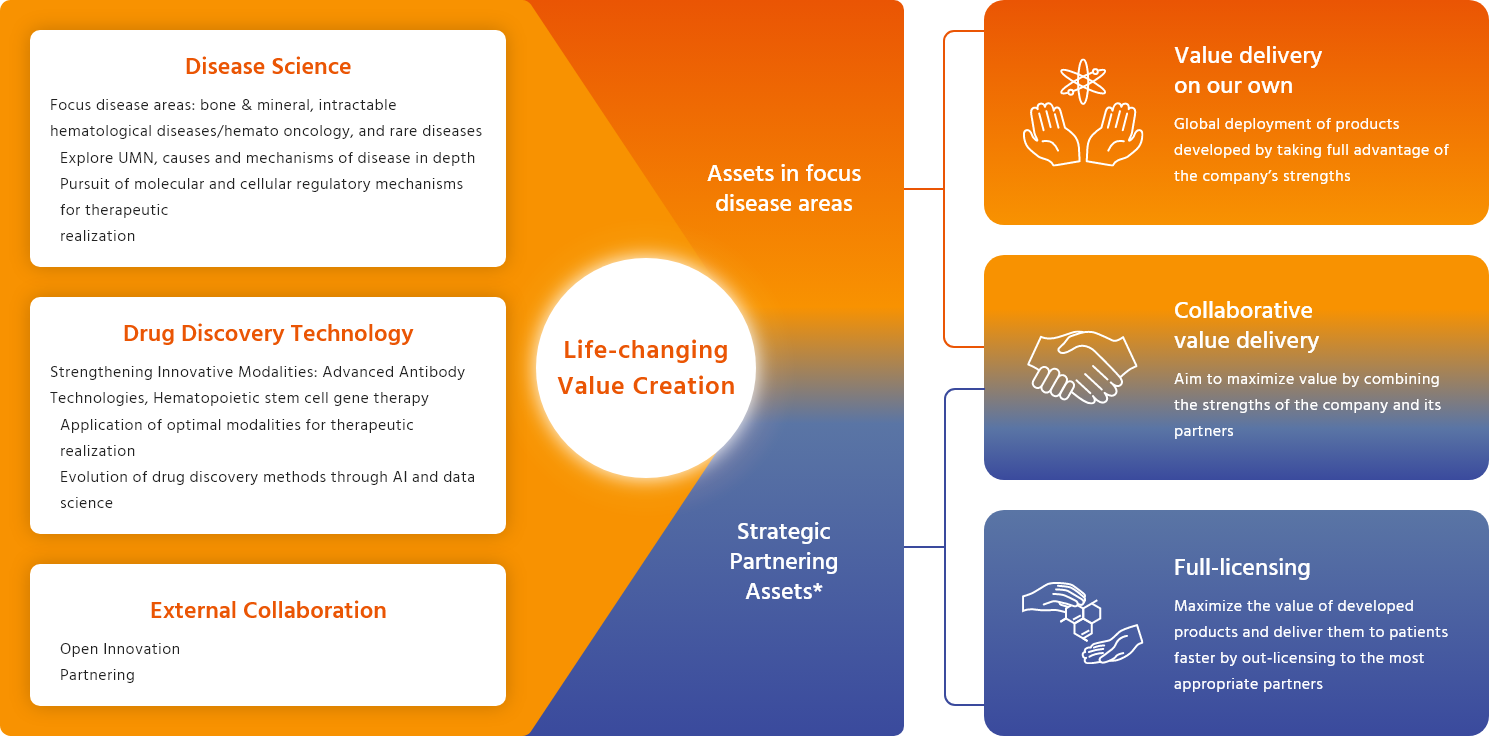
-
*Assets outside of the disease areas of focus are designated as strategic partnering assets, and value maximization is achieved through collaboration with partners.
DEVELOPMENT
PIPELINE
Click to see the up-to-date list of new drug candidates that Kyowa Kirin is developing with its cutting-edge technologies.
-
phase 1 11
-
phase 2 4
-
phase 3 5
In total 20 Programs
PRODUCTION AND
QUALITY ASSURANCE
We cultivate human capital with a global outlook and advanced expertise,
while maintaining facilities and systems that comply with GMP,
the international standard for pharmaceutical manufacturing and quality control.
By consistently delivering reliable, high-quality pharmaceuticals, we bring smiles to patients around the world.
R&D STRUCTURE
-
Drug Discovery Process
Kyowa Kirin’s drug discovery is divided into eight processes to maximize the integration of all technologies, knowledge,
and know-how from research and development to manufacturing. -
Global R&D Structure
Our Research and Development functions collaborate closely to advance global drug discovery initiatives.
This section introduces our collaborative structure from a global perspective.