Even if it is just one of 100,000 doses of medicine for us, for the patient, that one dose is everything.
We believe that developing new technologies that create life-changing value, promoting development of pharmaceuticals and ensuring the stable supply of high-quality pharmaceuticals are vital for a pharmaceutical company. We believe it is extremely important for each employee to have a sense of responsibility for “continuing to make people smile.”
Chief Supply Chain Officer (CSCO) &
Global Manufacturing Head
Toshiyuki Kurata

Committed to high quality, safety and compliance policies.
Our Mission is to assure safe, effective, and high-quality products and services by executing our strategic Global Quality Roadmap. We are committed to quality, compliance, and integrity, empowering our teams to create value for stakeholders and build trust with our patients.
Global Quality Assurance Head Jonathan Patroni

TOPICS
Kyowa Kirin's production in numbers
-
Production bases
2Plants
-
Production-related laboratories
2Bases
Biopharmaceuticals, Small molecule drugs
-
Cultivation tank size (maximum)
at a scale of 12,000 L
-
History of manufacturing
biopharmaceuticalsover30years
-
Renewable energy usage rate
100%
Domestic plants, electricity
-
New drug development
18programs
The best teamwork to achieve stable production
World-standard Quality Assurance system
Pharmaceuticals are products that directly affect human lives. At a pharmaceutical company, quality is the responsibility of the company as well as every employee. Without robust quality and compliance, we cannot retain the trust of patients, healthcare professionals, national regulatory authorities nor that of society. Kyowa Kirin’s Global QA Function is responsible for confirming that all processes involved in the production of products and investigational drugs are conducted properly and in compliance with relevant laws and regulations. In this way, the function fulfills its role of ensuring that there are no problems with the quality of the drugs delivered to patients.
Globally integrated quality management system
Kyowa Kirin's uses an eQMS (enterprise/electronic quality management system) globally. This system covers a wide range of quality assurance operations including change management, deviation control, corrective action/preventive action (CAPA), training, document management, auditing and supplier management by means of a complete electronic process. We now have in place a platform that enables us to meet a globally consistent standard as well as the requirements of relevant laws and regulations in each country.
A highly efficient production system
which supports a stable supply
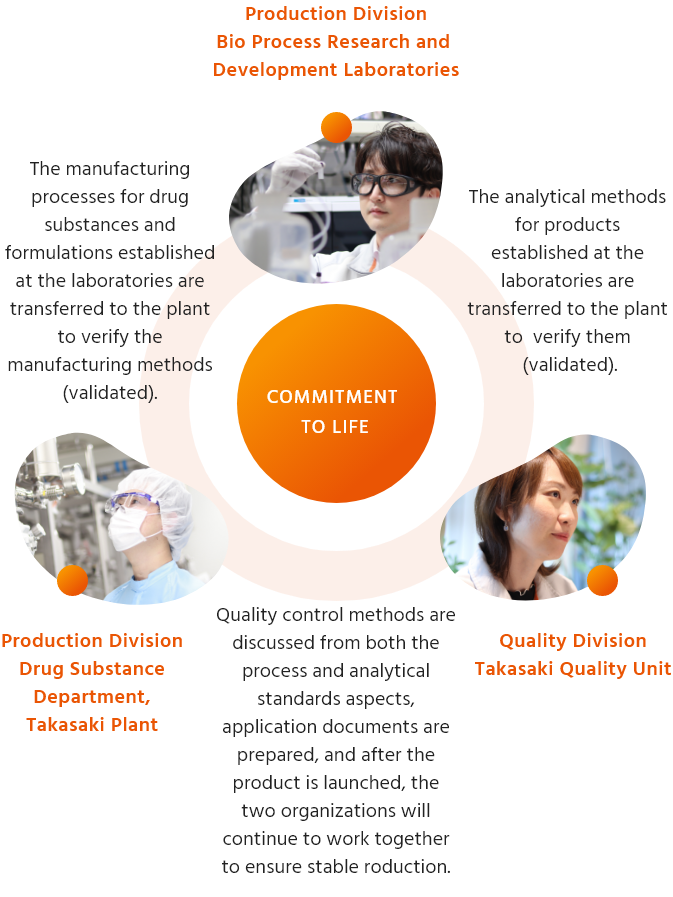
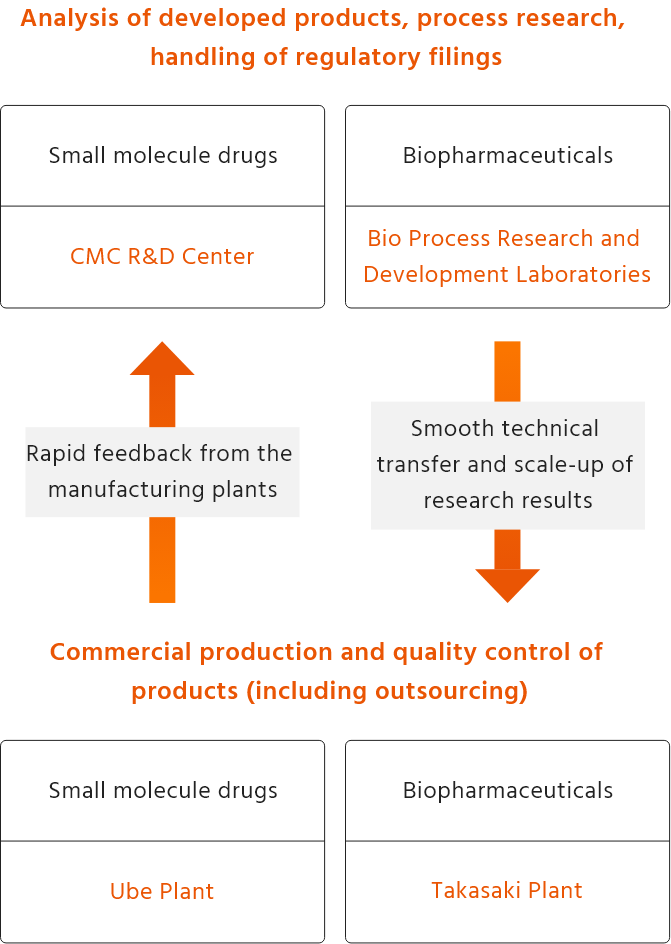
We achieve rapid market launch and stable supply of high-quality products throughout their lifecycle, from the development stage to post-market launch. Also, we will continue to deliver high-quality products by continuing to address drug fostering and evolution and research and development of new technologies. To achieve this, we work closely with various functions, including those involved in production technology development, manufacturing, analytical technology research and manufacturing approval applications, in order to take on the challenge of developing high-quality pharmaceuticals.
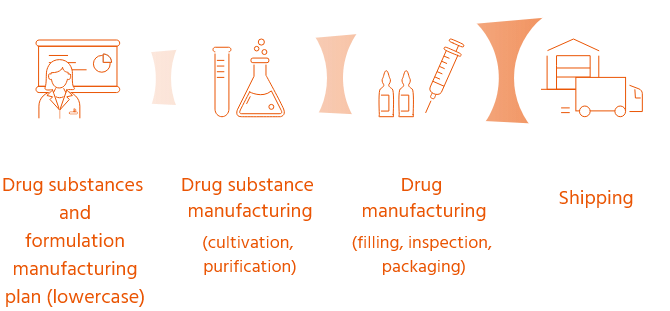
Flow up to product shipment
Kyowa Kirin’s production of biopharmaceuticals
As biopharmaceuticals are heterogeneous molecules, outstanding technologies are required to control their quality. Kyowa Kirin uses production and analytical technologies accumulated since it started the production of erythropoietin to produce and supply many biopharmaceuticals, including antibody drugs. In recent years, in addition to developing biopharmaceuticals that utilize our unique antibody technologies and protein engineering, we have developed new technologies such as single-use technology and continuous manufacturing technology aimed at making our plants even more productive.
Kyowa Kirin’s production of small molecule drugs
In the manufacturing of active pharmaceutical ingredients for small molecule drugs, we not only pursue high-quality, but also explore highly efficient synthesis routes and build manufacturing processes. During the production stage, dosage forms which are most suitable for patients are selected and raw materials and manufacturing conditions are optimized to further improve the quality of the formulation. In addition, we are committed to contributing to patients and people who support them by applying formulation technologies that add value such as ease of administration and handling (such as orally disintegrating tablets, bitterness masking, dividable tablets and granules and dry syrups for children) and utilizing formulation technologies that maximize the value of drugs (such as dissolution control technology, solubility improvement technology, and targeting technology).
?What are biopharmaceuticals?
Drugs that utilize proteins and other biomolecules with high pharmacological activities. Because of their high affinity for targets, they are expected to be highly effective with fewer side effects.
?What are small molecule drugs?
Drugs with relatively small molecules produced by chemical synthesis.
Products
Kyowa Kirin is a Japan-based Global Specialty Pharmaceutical Company, contributing to human health and well-being worldwide through innovative drug discovery and global commercialization, driven by state-of-the-art antibody technologies, in the core therapeutic areas of bone & mineral, intractable hematological diseases/hemato oncology, and rare disease.
Kyowa Kirin's product portfolio may not be available in all countries or regions, or may be approved for different indications, in different dosages, or in different strengths. In general, those products are available only by prescription through local healthcare professionals.
Please visit our local country or region website for local information: