Access to Medicines
Policy and Strategy
Kyowa Kirin considers improving access to medicines an important social issue related to health and welfare and is implementing various initiatives to help ensure patients have access to our medicines.
Kyowa Kirin Group Policy for Access to Medicines
We at the Kyowa Kirin Group seek to fulfill our management philosophy, “The Kyowa Kirin Group companies strive to contribute to the health and wellbeing of people around the world by creating new value through the pursuit of advances in life sciences and technologies.”
The Kyowa Kirin Group, as a global specialty pharmaceutical company originating from Japan and committed to continuously creating life-changing value that ultimately makes people smile, believes that improving access to medicines is an important social issue related to health and well-being.
The Kyowa Kirin Group believe that it is our mission to deliver medicines to as many patients as possible and as quickly as possible, and we will promote initiatives from three perspectives of "providing medicines that meet unmet medical needs," "improving access to medicines," and "Quality Assurance and Stable Supply and Ensuring Patient Safety and Appropriate Use of Medicines" by clarifying the necessary functions for each initiative, appointing managers to oversee each function, and promoting close cooperation among the functions as well as collaboration with external stakeholders.
This group policy applies to all employees of the Kyowa Kirin Group. We will also encourage all of our partners in the supply chain to act in the spirit of this policy.
Provide pharmaceuticals for Unmet Medical Needs
- Promotion of research and development for the creation of groundbreaking drugs
The Kyowa Kirin Group has been working to create new drugs for diseases for which there are no effective treatments, including rare diseases, by focusing on their pathological mechanisms. The Kyowa Kirin Group will continue to take on the challenge of advancing our antibody technology, as well as making full use of even more diverse modalities in our drug discovery efforts, leveraging Kyowa Kirin's strengths. We will also be utilizing open innovation to accelerate this R&D effort. The Kyowa Kirin Group believes that the most important factor in improving access to medicines is to provide patients with the medicines produced in this way.
Improving access to medicines
- Access to unapproved drugs
For patients with serious or life-threatening symptoms for whom no other effective treatment is available, the Kyowa Kirin Group will appropriately consider how to provide the investigational drug in accordance with the regulations of each country if the physician judges that the expected efficacy of the investigational drug outweighs the safety risks to the patient, even when the patient does not meet the criteria for participation in a clinical trial. - Intellectual property
Research and development are the business foundation of the Kyowa Kirin Group, and intellectual property is an important business asset for us. In countries with unique economic constraints, however, consideration will be given to patent applications and enforcement of patent rights. - Disease awareness
The Kyowa Kirin Group will work globally with medical professionals, academic institutions, patient groups, and others to promote disease awareness and patient advocacy activities in accordance with country regulations, with the objective of improving understanding of the disease. - Patient support
If safe use of quality-assured products is possible, the Kyowa Kirin Group will work to improve access to medicines through a variety of initiatives in accordance with national regulations, including programs to help patients who have difficulty obtaining medicines.
Quality Assurance and Stable Supply and Ensuring Patient Safety and Appropriate Use of Medicines
The Kyowa Kirin Group strives to ensure a stable supply through efficient global supply chain management, product quality assurance, and ensuring patient safety and appropriate use of medicines so that patients around the world can continue to use our products with peace of mind, and we deliver necessary medicines and related information to patients in an appropriate timing.
Indicators and Targets
In major markets, medicines need to be approved for marketing by regulators (FDA, PMDA, EMA) and then approved for reimbursement or coverage through different processes and agencies – Health Technology Assessments (HTAs) in EMEA, insurers and health plans in the US. The first priority is to increase the number of countries worldwide where our products are available in order to help treat more patients.
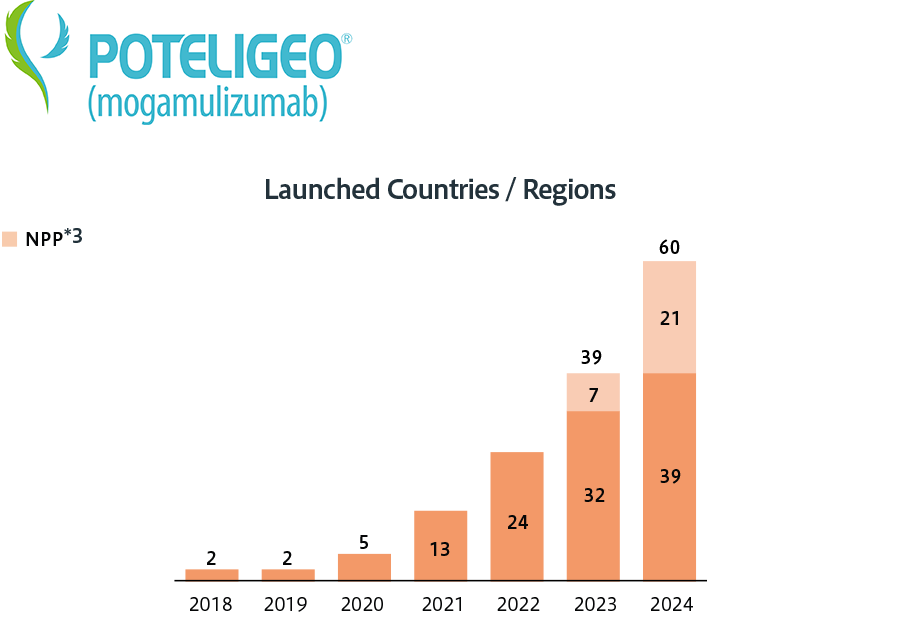
Kyowa Kirin discloses the number of countries where Crysvita and Poteligeo have been launched and the number of patients treated with Crysvita as indicators of how we are expanding access to our medicines.
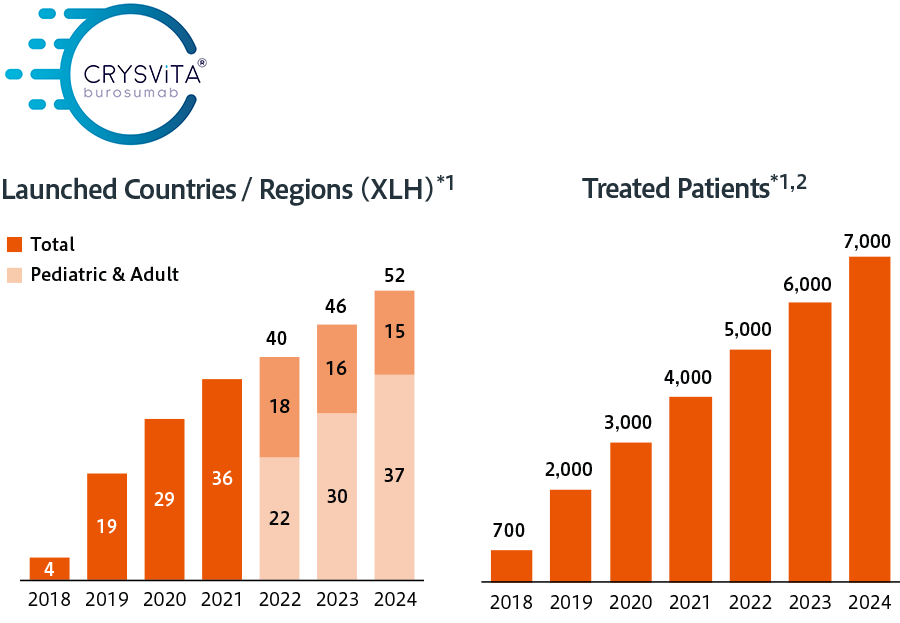
Target number of countries/regions across the world: 50 or more by 2025
- *1Excludes Latin America and Turkey, where Ultragenyx records sales
- *2The numbers of treated patients is an approximate number based on our calculations.
- *3Named Patient Program: The program that provides unapproved medications to patients with specific medical conditions who are not eligible for clinical trials or for whom other treatments have proven ineffective.
Specific Initiatives
Provide Pharmaceuticals for Unmet Medical Needs
Throughout our history, Kyowa Kirin Group has worked to create new medicines for diseases for which there are few effective treatments, including rare diseases, by focusing on the pathological disease mechanism. And we continue to pursue new drug discoveries utilizing innovative modalities, such as advanced antibody technology, cell therapy and hematopoietic stem cell gene therapy, to provide Life-changing value and help more patients around the world.
Globally, Kyowa Kirin markets Crysvita®, Poteligeo®,and Nouriast®/Nourianz® and Libmeldy®/Lenmeldy®. In addition, Kyowa Kirin identified bone & mineral, intractable hematological diseases/hemato oncology, and rare diseases as our focus areas for new research and development. In these areas, we aim to create life-changing value through the utilization of innovative modalities described above and external collaborations, as well as through in-depth exploration of unmet medical needs and the causes and mechanisms of diseases. In addition, based on our Group Policy for Access to Medicines, established in 2022, Kyowa Kirin strives to improve access to our medicines across the value chain.
Improving access to medicines
Disease awareness
In the case of rare diseases and other conditions that are difficult to diagnosis, early diagnosis is particularly important, and disease awareness activities can help ensure greater understanding and action by physicians, patients and patient groups. In addition, Kyowa Kirin recognizes that patients may face a number of challenges throughout the patient journey,* especially patients living with rare diseases.
Kyowa Kirin contributes to disease awareness by delivering disease-related information to patients and their families, healthcare professionals, and other relevant stakeholders. In addition to disease-related information, Kyowa Kirin also strives to raise awareness of the challenges faced by patients and their families in order to deepen their understanding. Please click on the links below for examples of disease awareness activities of the Kyowa Kirin.
Shine A Light on XLH(English、Japanese![]() )the CTCL Global Care Collaborative’s Time to Act global consensus statement(English)
)the CTCL Global Care Collaborative’s Time to Act global consensus statement(English)
Patient support
As described in the index for access to medicines, our first priority is to increase the number of countries worldwide where our products are available so that Kyowa Kirin provide Life-changing value and make people smile. However, due to factors such as different regulatory regimes in each market, even if effective drugs are available, patients may not be able to gain access because of market conditions specific to their country.
To address this issue, Kyowa Kirin is working to provide medicines upon request from physicians after determining eligibility.
For example, after clinical trials are completed and marketing approval has been obtained, the Early Access Program ensures access to medicines until reimbursement approval. Also, in countries where we have no plans to gain authorization, the Named Patient Program is provided.
In addition, where needed, Kyowa Kirin works with various stakeholders to overcome access challenges through programs including compassionate use, supports with healthcare coverage challenges, and other options as allowed in each market.
Quality Assurance and Stable Supply, and Ensuring Patient Safety and Appropriate Use of Medicines
For related initiatives, please see the following pages.
Other related initiatives
Participating in the Global Health Innovative Technology Fund (GHIT Fund)
Since 2016, Kyowa Kirin has been participating in the GHIT Fund, a public-private partnership for promoting discovery of new drugs for infectious diseases in developing countries.
A public-private partnership jointly funded by the Government of Japan, Japan’s leading life science companies, the Bill & Melinda Gates Foundation, the Wellcome Trust, and the UN Development Programme (UNDP), the GHIT Fund is the world’s first of its kind to exclusively tackle product development in the global health field.
By endorsing the GHIT Fund’s objective of establishment and activities and by supporting its activities to deliver new drugs to patients with currently untreatable diseases, We aims at contributing to the enhancement of global health.
June 6, 2016 Notification on participation in GHIT Fund (in Japanese only)![]()
June 2, 2017 Notification on participation in GHIT Fund’s second phase (in Japanese only)![]()
July 3, 2023 Notification on participation in GHIT Fund’s third phase (in Japanese only)![]()
Efforts to Reduce the Medical Costs Burden of Biopharmaceuticals
As public health spending increase, curbing them has become a issue in many countries.
The Kyowa Kirin Group, together with Kyowa Kirin Frontier Co., Ltd. and FUJIFILM KYOWA KIRIN BIOLOGICS Co., Ltd. is committed to contribute to society by responding to social demands to curb health spending and increasing opportunities for more patients to receive treatment.
For details, please visit the each company's website.